Paul is the Head of Medical Excellence, Operations and Governance at Novartis, with responsibility for defining and governing enterprise-wide medical standards and policies, ensuring timely clinical trial disclosure and transparency; and the oversight, standards, training, systems and operations for compassionate use/expanded access, scientific grants, peri- and post-registration medical programs (e.g. Investigator initiated trials, research collaborations, non-interventional studies, registries, and patient support/assistance programs).
He has over 20 years’ experience in the Pharma industry across multiple therapeutic areas and roles. Prior to joining the pharmaceutical industry, he practiced as a clinical pharmacist, and is trained in law, business, bioethics, and general management. He previously served as an industry representative over a 5-year period on the WHO/Roll-Back Malaria (RBM) case management working group and has managed various partnerships (Public-Private & Private-Private).
Paul is a member of the Royal Pharmaceutical Society of Great Britain (MRPharmS). He has a Doctorate in Clinical Pharmacy from the University of Florida, a first-class honors master’s degree in Pharmacy from the Sunderland School of Pharmacy in the UK, a qualifying Law degree from the College of Law in London (now University of Law), certificates in Global Health Delivery and Public Leadership from Harvard University, a Fellowship in Bioethics from the Harvard Medical School, and an executive MBA from the International Institute for Management Development (IMD) in Lausanne, Switzerland.
He is a regular speaker/panelist at conferences on Global Health, Patient Access, Medical Governance, and also publishes on these topics.


 Effective March 1, 2025, Thomas J. Vilsack, former United States Secretary of Agriculture and Governor of Iowa, became the first Chief Executive Officer for the World Food Prize Foundation. In this new role, Governor Vilsack is focusing on expanding the Foundation’s global network, and will further position the Foundation as a leader in addressing global food and nutrition insecurity, continuing his lifetime of public service.
Effective March 1, 2025, Thomas J. Vilsack, former United States Secretary of Agriculture and Governor of Iowa, became the first Chief Executive Officer for the World Food Prize Foundation. In this new role, Governor Vilsack is focusing on expanding the Foundation’s global network, and will further position the Foundation as a leader in addressing global food and nutrition insecurity, continuing his lifetime of public service. Shelby Coffey III is a distinguished journalist, media executive, and thought leader whose career has helped shape the landscape of American news and public discourse. Over several decades, Coffey has held some of the most influential roles in journalism, including serving as editor of the Los Angeles Times, executive vice president of ABC News, and deputy managing editor of The Washington Post. His editorial leadership extended to key roles as president of CNN Financial News, editor of the Dallas Times Herald, and U.S. News & World Report.
Shelby Coffey III is a distinguished journalist, media executive, and thought leader whose career has helped shape the landscape of American news and public discourse. Over several decades, Coffey has held some of the most influential roles in journalism, including serving as editor of the Los Angeles Times, executive vice president of ABC News, and deputy managing editor of The Washington Post. His editorial leadership extended to key roles as president of CNN Financial News, editor of the Dallas Times Herald, and U.S. News & World Report. Jerry Steiner has spent 40 years involved in agriculture following growing up on a Wisconsin dairy farm. He began his career with Monsanto, in multiple business leadership roles. From 2003-2013 he served as a member of the Executive team, as the company’s Executive Vice President of Sustainability and Corporate Affairs. He led the company’s global Government, Public and Industry Affairs teams across the 70 countries where Monsanto conducts business. This experience got Jerry connected to the Keystones centers work in agriculture. Key among his responsibilities were shaping the company’s public policy and building partnerships aimed at helping farmers around the world produce more food, while conserving valuable resources like water and energy. Two unique partnership that developed under his leadership were drought tolerant corn with 5 African countries, CIMMYT and the Gates foundation, and a building a sustainable business model in Brazil with the value chain leading to significant multi-company investment and soybean varieties that can protected themselves.
Jerry Steiner has spent 40 years involved in agriculture following growing up on a Wisconsin dairy farm. He began his career with Monsanto, in multiple business leadership roles. From 2003-2013 he served as a member of the Executive team, as the company’s Executive Vice President of Sustainability and Corporate Affairs. He led the company’s global Government, Public and Industry Affairs teams across the 70 countries where Monsanto conducts business. This experience got Jerry connected to the Keystones centers work in agriculture. Key among his responsibilities were shaping the company’s public policy and building partnerships aimed at helping farmers around the world produce more food, while conserving valuable resources like water and energy. Two unique partnership that developed under his leadership were drought tolerant corn with 5 African countries, CIMMYT and the Gates foundation, and a building a sustainable business model in Brazil with the value chain leading to significant multi-company investment and soybean varieties that can protected themselves. Jennifer Morris is the Chief Executive Officer of The Nature Conservancy, leading a team of nearly 6,000 staff working in more than 80 countries and territories tackling the dual crises of the
Jennifer Morris is the Chief Executive Officer of The Nature Conservancy, leading a team of nearly 6,000 staff working in more than 80 countries and territories tackling the dual crises of the  Congressman Joe Neguse represents Colorado’s 2nd District in the U.S. House of Representatives. He was elected to his first term in November 2018, becoming the first Black Member of Congress in Colorado history. In December 2022, Rep. Neguse was elected by his colleagues to serve as Chair of the Democratic Policy and Communications Committee (DPCC), becoming the first Coloradan to serve in a senior elected leadership role in the House in over 85 years. He serves on the Natural Resources and Judiciary Committees, and was also appointed by House Minority Leader Hakeem Jeffries to serve as one of four Democrats on the prestigious Rules Committee. Rep. Neguse serves as Ranking Member on the House Subcommittee on Federal Lands, which he previously Chaired in the 117th Congress.
Congressman Joe Neguse represents Colorado’s 2nd District in the U.S. House of Representatives. He was elected to his first term in November 2018, becoming the first Black Member of Congress in Colorado history. In December 2022, Rep. Neguse was elected by his colleagues to serve as Chair of the Democratic Policy and Communications Committee (DPCC), becoming the first Coloradan to serve in a senior elected leadership role in the House in over 85 years. He serves on the Natural Resources and Judiciary Committees, and was also appointed by House Minority Leader Hakeem Jeffries to serve as one of four Democrats on the prestigious Rules Committee. Rep. Neguse serves as Ranking Member on the House Subcommittee on Federal Lands, which he previously Chaired in the 117th Congress.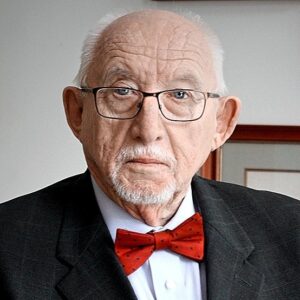 Llewellyn King was born in Southern Rhodesia, now Zimbabwe. He went into journalism as soon as he turned 16, stringing for Time magazine and United Press in Africa.
Llewellyn King was born in Southern Rhodesia, now Zimbabwe. He went into journalism as soon as he turned 16, stringing for Time magazine and United Press in Africa. Steven Williams is the Chief Executive Officer of PepsiCo North America, overseeing a more than $48 billion business that spans PepsiCo’s Foods and Beverage operating units. His leadership encompasses more than 125,000 associates and over 900 locations across the U.S. and Canada. Steven joined PepsiCo in 2001 as part of PepsiCo’s acquisition of the Quaker Oats Company, which he joined in 1997, and has held leadership positions of increased responsibility since.
Steven Williams is the Chief Executive Officer of PepsiCo North America, overseeing a more than $48 billion business that spans PepsiCo’s Foods and Beverage operating units. His leadership encompasses more than 125,000 associates and over 900 locations across the U.S. and Canada. Steven joined PepsiCo in 2001 as part of PepsiCo’s acquisition of the Quaker Oats Company, which he joined in 1997, and has held leadership positions of increased responsibility since.